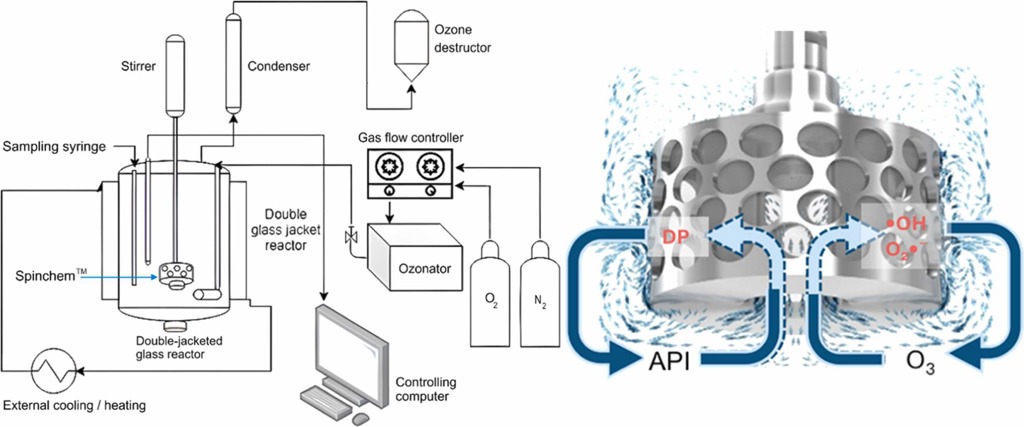
"Removal of pharmaceuticals from wastewater remains a major environmental challenge, requiring efficient and selective Advanced Oxidation Processes (AOPs). Catalytic and non-catalytic ozonation was investigated in a laboratory-scale reactor under optimized flow conditions (500-750 mL min⁻¹, 98% O₂ feed). Ozonation kinetics of active pharmaceutical ingredient mixtures (APIs) consisting of ibuprofen (IBU), diclofenac (DCF), carbamazepine (CBZ), sulfadiazine (SDZ), and sulfamethoxazole (SFX) (40 mg L⁻¹ each) —was investigated using iron-modified zeolite catalysts, Fe-H-Y and Fe-H-Beta, under semi-batch operations (0.5 g catalyst, 20 °C) in order to correlate degradation and mineralization efficiency with catalyst structure, acidity, and stability. Both catalysts significantly improved the ozone utilization compared to non-catalytic ozonation. Interestingly, Fe-H-Y accelerated initial degradation rate, while the use of Fe-H-Beta resulted in the highest level of mineralization. Adsorption–desorption analysis revealed that the molecular size and polarity controlled the interactions between the pharmaceutical and the catalyst: smaller polar compounds (SDZ, SFX) exhibited stronger adsorption on the catalyst, while bulkier molecules (DCF, IBU) were restricted to external surfaces. Post-reaction characterization confirmed that the Fe-H-Y retained more surface area and exhibited lower Fe leaching, while Fe-H-Beta showed significantly higher carbon deposition. Overall, Fe-H-Y combined rapid kinetics and structural stability, while Fe-H-Beta provided higher mineralization, at the expense of more extensive fouling. The study demonstrated that optimized ozonation conditions, coupled with tailored zeolite catalysts, markedly improve the oxidation efficiency and long-term performance in the oxidation of pharmaceuticals."
Keywords
Catalytic ozonation, Advanced oxidation processes, Emerging contaminants, Pharmaceutical mixtures, Zeolites, Wastewater treatment, Transformation products, Reaction kinetics
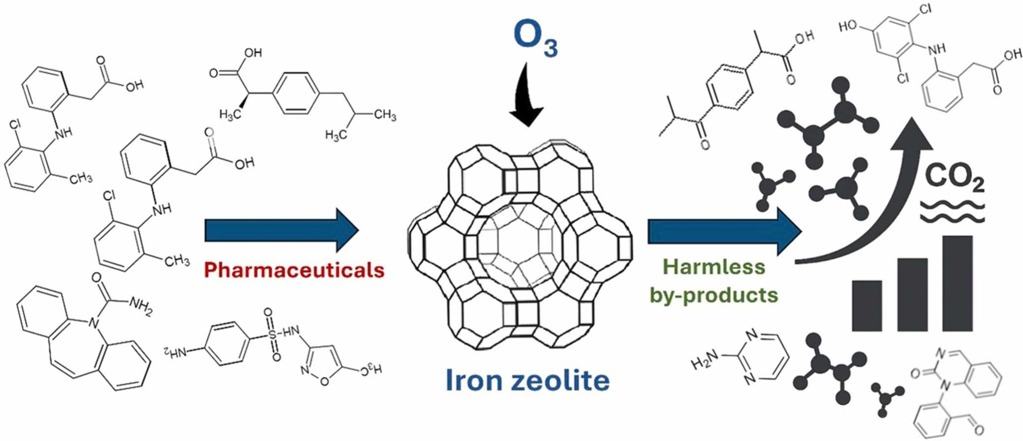
Process diagram showing pharmaceutical compounds transformed by ozone and iron zeolite catalyst into harmless by-products and CO2, with increasing bar chart indicating mineralization efficiency.
Highlights:
- Rotating bed reactor system enabled fast pharmaceutical degradation: Experiments used the SpinChem® rotating bed reactor (RBR) to confine the catalysts and provide efficient mass transfer.
- Complete pharmaceutical removal within 7–8 minutes: All five pharmaceutical compounds (ibuprofen, diclofenac, carbamazepine, sulfadiazine, sulfamethoxazole) were fully removed using the Fe-H-Y catalyst in the rotating bed reactor system.
- Catalysts increased mineralization by up to 42%: Total organic carbon (TOC) removal reached 82–89% with Fe-modified zeolite catalysts compared to 63% without catalyst.


