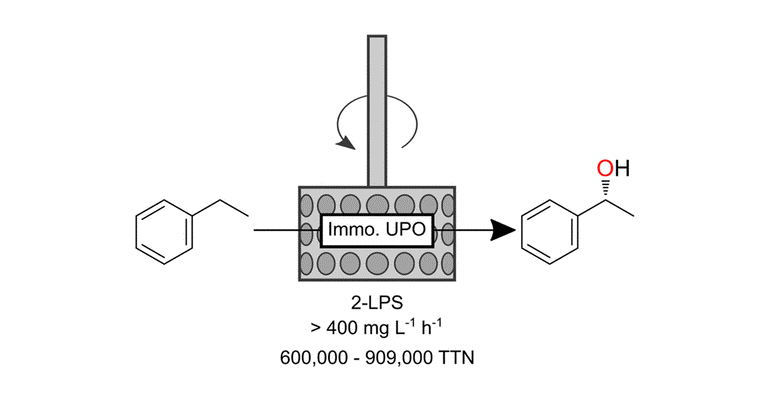
“The ability of unspecific peroxygenase (UPO) to hydroxylate a wide range of substrates with just H2O2 as a cosubstrate has attracted a great deal of attention in biocatalytic research. The enzyme’s intrinsic limitation to be inactivated by excess amounts of the oxidative cosubstrate has been tackled with in or ex situ hydrogen peroxide (H2O2) provision strategies. In this paper, we present the application of the covalently immobilized UPO mutant PaDa-I in a rotating bed reactor for the hydroxylation of ethylbenzene in a two-liquid-phase system. By monitoring product formation in the organic phase and H2O2 concentration in the aqueous phase, the multiphasic reaction was optimized.”
Highlights:
- “The results presented in this work show that the path for industrial and economic use of UPOs is becoming clearer, combining high productivities and TTNs with the ease of operation in an RBR.”
- “In a classic fed batch, 414 mM target product was accumulated in the organic phase over 58 h. A productivity of 436 mg/(L*h) and a TTN of 658 000 are, to our knowledge, among the highest ever reported for UPO-catalyzed hydroxylation of ethylbenzene in a 2LPS.” (2LPS = two-liquid phase system)
- “In conclusion, we present the first published use of an immobilized UPO in an RBR to produce (R)-1-phenylethanol."
Authors & Research Group
This research was conducted by an international collaboration between Aarhus University in Denmark and SpinChem AB in Sweden, with contributions from enzyme immobilization specialists, bringing together expertise in biocatalysis, enzyme engineering, and rotating bed reactor technology.
Principal Investigator:
Selin Kara – Prof. Dr.-Ing. habil., Department of Biological and Chemical Engineering, Aarhus University, Denmark. Research focus: biocatalysis, enzyme technology, process engineering, and sustainable biotransformations.
Team members:
- Markus Hobisch – Biocatalysis and Bioprocessing Group, Department of Biological and Chemical Engineering, Aarhus University
- Piera De Santis – Biocatalysis and Bioprocessing Group, Aarhus University
- Simona Serban – Enzyme immobilization technology
- Alessandra Basso – Enzyme immobilization technology
- Emil Byström – SpinChem AB, Umeå, Sweden
SpinChem Perspective
This study demonstrates what we see as a practical turning point for peroxygenase-based processes. The challenge of enzyme inactivation by hydrogen peroxide has long limited the industrial adoption of UPO enzymes, and this work shows that a rotating bed reactor can address that limitation head-on.
For pharmaceutical manufacturers working with selective hydroxylation reactions, the ability to achieve productivities of 436 mg/(L·h) while improving selectivity from 62% to 79% through simple repetitive batch operation is significant. Higher selectivity means less overoxidation to the acetophenone byproduct, and the enzyme is easily recycled between batches without disassembling the reactor.
For producers of enantiopure alcohols like (R)-1-phenylethanol, this approach brings the industrial and economic use of UPOs closer to reality, as the authors themselves conclude. The fact that the immobilized enzyme showed negligible leaching and excellent reusability across multiple cycles makes it relevant for any setting where consistent biocatalytic performance and efficient use of enzyme resources matter.
What makes this work particularly interesting from a process perspective is the simplicity of the RBR setup. Switching between batches required nothing more than draining, rinsing, and refilling the vessel, with the enzyme remaining in place. That kind of operational ease is what makes the transition from lab proof-of-concept to production-scale implementation realistic.
—Erik Löfgren, CTO SpinChem AB

