Biocatalysis, the future of sustainable chemistry
Enzymes display remarkable chemo-, regio-, and stereoselectivity. The identification of a suitable enzyme that converts the mandated substrate to the desired product isomer, allows to significantly shorten reaction pathways compared to traditional multistep synthesis. Notably, protection and deprotection strategies can often be omitted, and difficult isomer separation may be avoided.
The mild conditions and modest temperatures employed in enzymatic reactions, in combination with the highly selective catalysis, typically result in few to no significant side reactions.
Additionally, environmental and toxicological benefits can be reaped by avoiding pathways that employ metal catalysts and hazardous reagents.
Exploring enzymes types and their applications
Enzymes are divided into seven classes according to the type of reaction they catalyze.
EC 1 - Oxidoreductases catalyze oxidation-reduction reactions, hence involving the transfer of electrons from one molecule to another.
EC 2 - Transferases catalyze the transfer of certain groups from one molecule to another.
EC 3 - Hydrolases catalyze the hydrolysis of various bonds.
EC 4 - Lyases catalyze the cleavage of various bonds by means other than hydrolysis, often forming a double bond or a new ring structure.
EC 5 - Isomerases catalyze the rearrangement of atoms within a molecule to form a structural isomer.
EC 6 - Ligases catalyze the formation of a new chemical bond, facilitated by the concommitant consumption of a high-energy cosubstrate.
EC 7 - Translocases facilitate the movement of ions or molecules across membranes or their separation within membranes.
Reaction optimization: Factors that influence the activity and stability of enzyme and improves your reaction
The activity and stability of enzymes are governed by several factors, including temperature, pH, solvent type or buffer conditions, water activity, immobilization, stirring, and reaction parameters. Understanding these factors allows us to optimize enzyme-catalyzed reactions and to improve their efficiency and effectiveness.
Temperature
Enzymes are subject to two competing processes: On the one hand an increase in temperature raises the catalytic turnover according to Arrhenius, and on the other hand it accelerates the breakdown of the enzyme. The combined result is a bell-shaped temperature-activity curve, i.e., enzymes have a specific optimal temperature with peak productivity flanked by diminishing returns at both higher and lower temperatures.
Enzymes are frequently engineered to shift the temperature range of optimal activity and to thereby make them more resistant to denaturation. The desired shift in temperature can be both to higher and lower temperatures to either allow for reactions at elevated temperatures or to ensure high activity even at low temperatures. Enzymes can also be engineered to allow for a broadened substrate scope or non-natural substrates and products. Finally, engineering may confer stability in non-conventional media.
pH
Similar to temperature dependence, enzymes show peak activity within a narrow pH range. When moving away from the optimal pH, the activity is diminished and finally lost. Consequently, some form of pH control is often necessary.
Solvent/buffer
Choice of buffer and buffer strength may provide pH control and stability in aqueous systems and consequently also determine activity and stability. In non-conventional solvents, pH effects are defined by the interplay of physicochemical properties of substrates, products, and the reaction medium. This may go so far as to render these effects completely void. However, solvents themselves may have a strong impact on enzyme stability and activity. If necessary, process analytical technology (PAT) and titration allow for active pH control in any case. Choice of solvent also strongly affects the enzymatic activity.
Water activity
Enzymes require a minimum amount of water to sustain their catalytic function. This is typically due to the need for structurally-bound water and becomes important for reactions in non-aqueous media. However, the share of water required is frequently less than 1% of the total reaction medium. As a consequence - and perhaps counter-intuitively - a small amount of water is also added to reactions that from a thermodynamic perspective should ideally be 'water-free' (i.e., condensation reactions).
Immobilization
Immobilization of free enzymes onto solid materials (enzyme carriers) often provides stabilization and thereby prolonged lifetime of the enzyme. However, it also affects enzymatic activity whereby an increase is the more desirable yet less prevalent result. The success of the immobilization is strongly defined by the compatibility between enzyme and enzyme carrier material, and trials with several different materials are advisable. Arguably, the most common enzyme carrier materials are synthetic polymers (resins), but inorganic materials such as glass, natural fibers, etc. are also used and bio-based organic alternatives gain traction as well. The final choice is typically based on overall catalytic productivity.
Inherently connected to the enzyme carrier material is the method of attachment. While some enzymes, notably lipases, may be attached non-covalently by hydrophobic interactions, covalent attachment is often needed. The chemistries used hereby are versatile and frequently react nucleophilic free amines or thiols from the enzyme surface with electrophilic reactive groups (epoxides, aldehydes) on the carrier surface. The exact choice of attachment strategy will definitely affect the catalytic properties of the obtained heterogenous biocatalyst preparation.
Read more in our insight 'Introduction to Enzyme Immobilization'.
Mixing
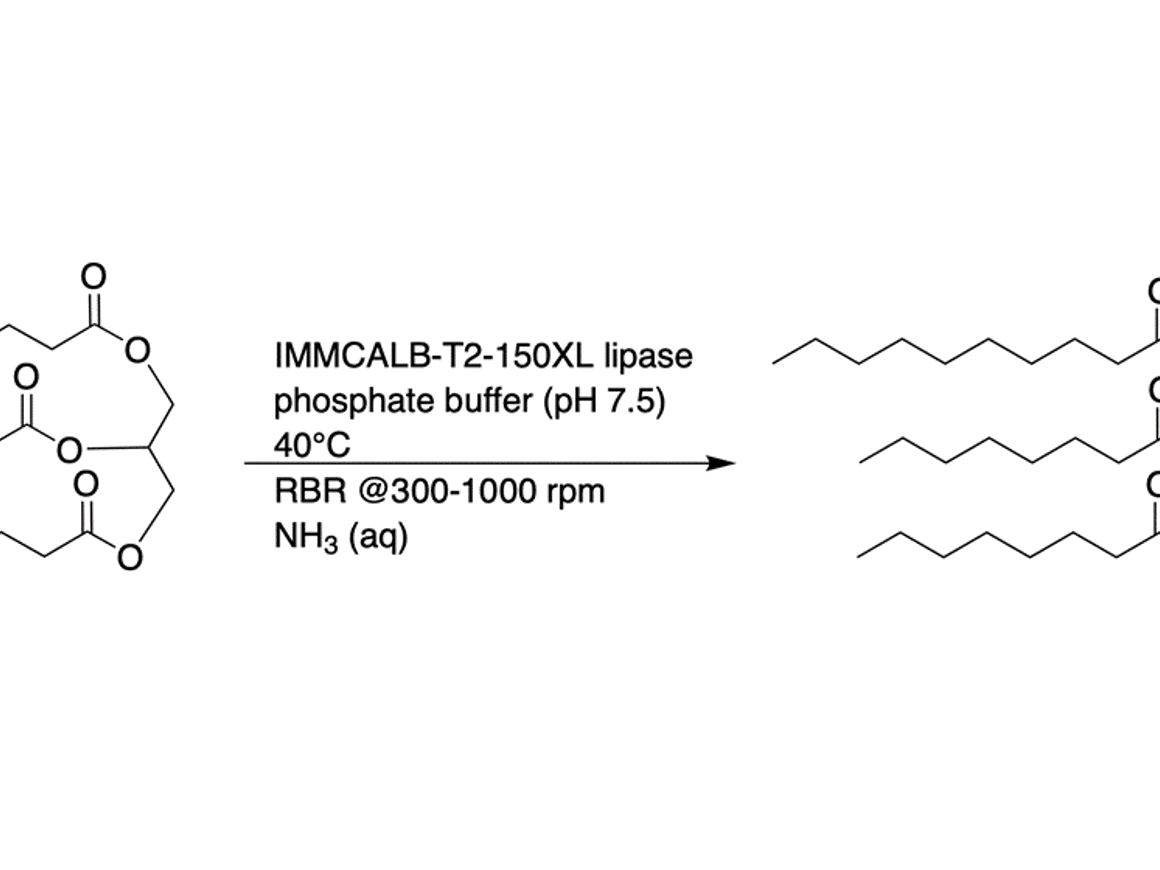
In the case of industrially preferred immobilized enzymes, the method of mixing determines the longevity of the heterogeneous catalyst. For batch reactors, the rotating bed reactor offers almost complete protection against the grinding effects that standard stirred tank reactors suffer from. As a result, extended recycling becomes feasible and convenient.

A rotating bed reactor (RBR) is designed to prevent disintegration of solid phase particles by retaining them inside a rotating cylinder during the reaction. This minimizes solid phase debris even at high speeds, which would normally occur due to mechanical forces when using stirred tank reactors. Due to this characteristic of the RBR, reaction rates can be improved by increasing the stirring speed without simultaneously shortening the lifespan of the solid particles. This ensures easy in-line monitoring of the process, as well as a more efficient recycling of the solid phase, making the RBR a cost and time efficient alternative to conventional methods for immobilized enzyme biocatalysis.
Reaction parameters
Perhaps more related to the efficiency of the enzymatic reaction than the stability of the enzyme are parameters such as reagent stoichiometry, gas introduction method, and the minimization of temperature or concentration gradients. Proper mixing ensures the necessary mass transfer of all substrates to the enzyme and is crucial especially in three-phase reactions where saturation with gaseous reagents has to be considered as well. Here too, the RBR provides important advantages over stirred tank reactors.