Many heterogeneous processes (e.g., adsorption or catalysis) are made faster by increasing the solid-to-liquid ratio. Studying scale-up effects can also help to predict full-scale performance. For these reasons, it is advisable to invest in equipment that can handle variations in operating conditions such as liquid volume, solids loading, pH, and temperature.
The RBR S3 Plus is the most modular rotating bed reactor for laboratory use. Made from two stacked rotating bed reactors, the S3 Plus quickly converts to a single RBR S3 for use with smaller solid loadings. When used in the dedicated glass reactor system, this yields an operating range of 250 - 1500 mL of liquid and 0 - 140 mL of solids.
This application note investigates the effect of solids loading on the reaction rate of two applications:
- dye adsorption
- biocatalytic esterification
These two reactions are mass-transport limited and relatively fast.
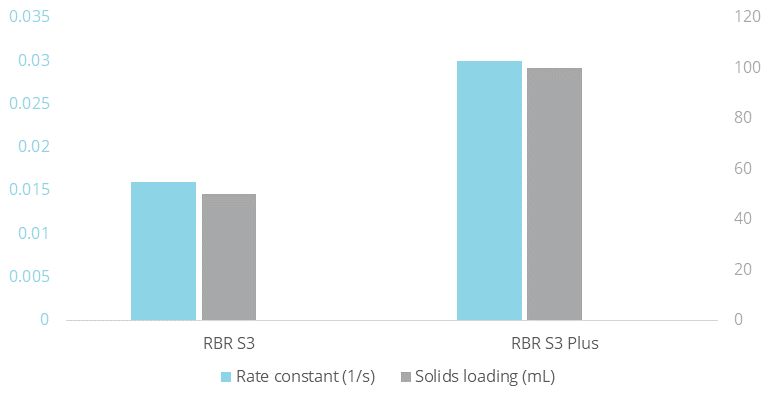
In the first case, an RBR S3 and an RBR S3 Plus were filled with 50 mL and 100 mL of the ion-exchange resin Purolite® NRW1160, respectively. Methylene blue was dissolved in water and the solution was decolorized by spinning an RBR at 600 RPM (reaction conditions in the details below). The results were clear: Each case followed 1st order kinetics with a rate constant for the RBR S3 Plus that was twice that of the RBR S3. Note that the solid-to-liquid ratio for the RBR S3 Plus was also twice that of the RBR S3.
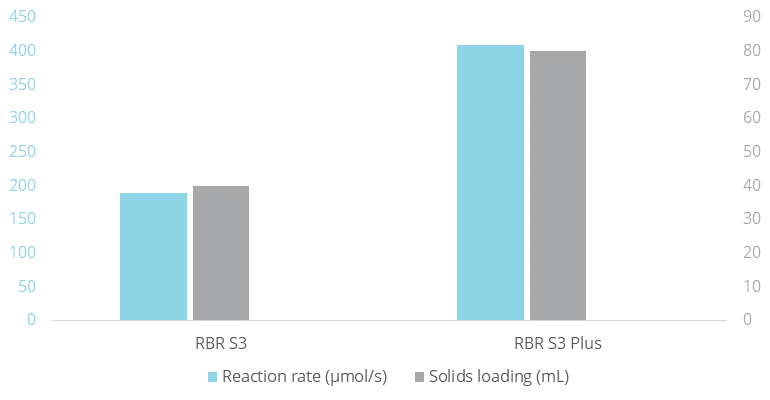
For the enzymatic esterification, the same rotating bed reactors (RBR S3 and RBR S3 Plus) were filled with 40 mL and 80 mL respectively of the biocatalyst Purolite® immo PS. The rotating bed reactors were used in separate reactions in mixtures of lauric acid, 1-propanol and water. Also in this case the reaction rate was proportional to the solid-to-liquid ratio, yielding twice the productivity with the RBR S3 Plus compared to the RBR S3.
The conclusion is that with a rotating bed reactor you are making the most out of the solid-phase. Doubling the amount of catalyst or adsorbent will generally double the reaction rate constant, which makes scaling up straightforward.
Contact us today to discuss how we can scale your process!



