
Is Water Affecting your Biocatalytic Reaction?
There are many industrial applications of biocatalysis such as esterification and amide formation that involve water as a by-product and also as a necessity to sustain enzymatic activity.
A good example for this is the enzymatic production of Capsaicin. The increasing concentration of water promotes the reverse reaction (hydrolysis), limiting yields and reaction rates.
Water: Good or Bad?
In short: both, hence it is a powerful parameter for reaction optimization. Research from Lund University showed that the effect of water activity on an enzymatic transesterification was two-sided: "Water is needed initially to start the reaction and maintain enzyme activity but has a negative effect on both the yield and product quality towards the end of the reaction." Find the article here.
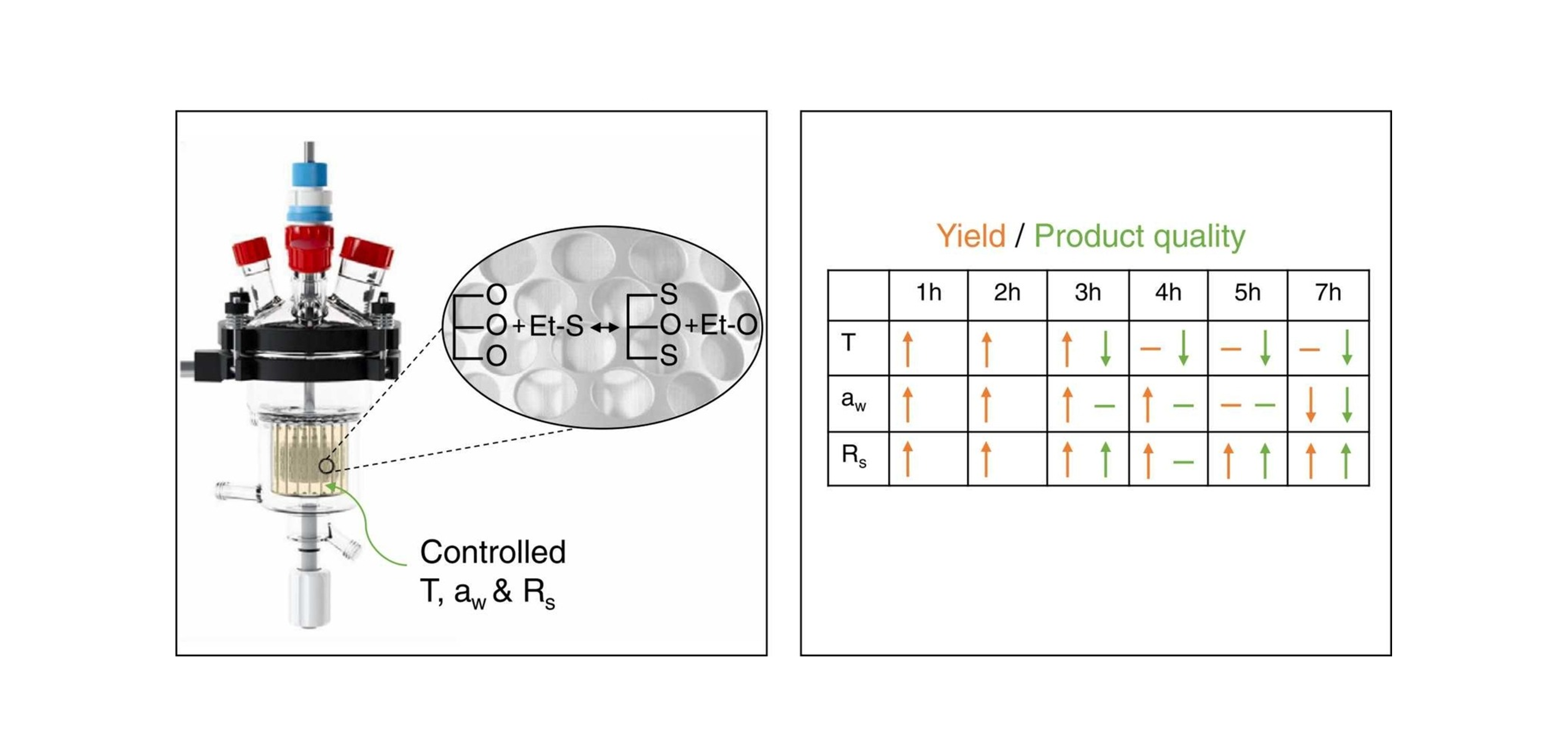
Removing Water
Challenges presented by water as a by-product can be overcome through continuous water removal. This maintains a thermodynamic equilibrium favourable for product formation. A common strategy to do so is evaporation, in which the water is cleared from the reaction mixture via the headspace. Hereby, a combination of heating and vacuum accelerates the process and allows for evaporation at temperatures much milder than 100 °C. For purposes like this, SpinChem's reactors are suited for connection to vacuum systems.

 The RBR simplifies downstream processing and makes solids such as molecular sieves feasible in applications they would otherwise contaminate.
The RBR simplifies downstream processing and makes solids such as molecular sieves feasible in applications they would otherwise contaminate.