In earlier work, the biocatalytic hydrolysis of medium chain triglycerides was successfully demonstrated in biphasic reaction. For this, the lipase from Thermomyces lanuginosus (TL) was immobilized on IB-COV-1 and effectively scaled from 0.3 L to 750 L by deployment of a SpinChem rotating bed reactor (RBR).
Following this success, a collaboration between Naturbeads, Bath University and ChiralVision led to the development of cellulose-based beads as alternative enzyme carriers for above application. These beads are characterized by high thermal and mechanical stability, uniform size, spherical shape, and high porosity (>90%), which makes them excellent biodegradable alternatives to classical plastic support beads. Moreover, versatile surface functionalization of these beads is possible and allows to control enzyme binding modes to adjust for good activity recovery and stable catalytic performance.
Experimental comparison of the same amount of TL lipase immobilized either on acrylic or cellulose beads underpinned the comparable if not better performance of the novel bio-based enyzme carriers when used for MCT oil hydrolysis:
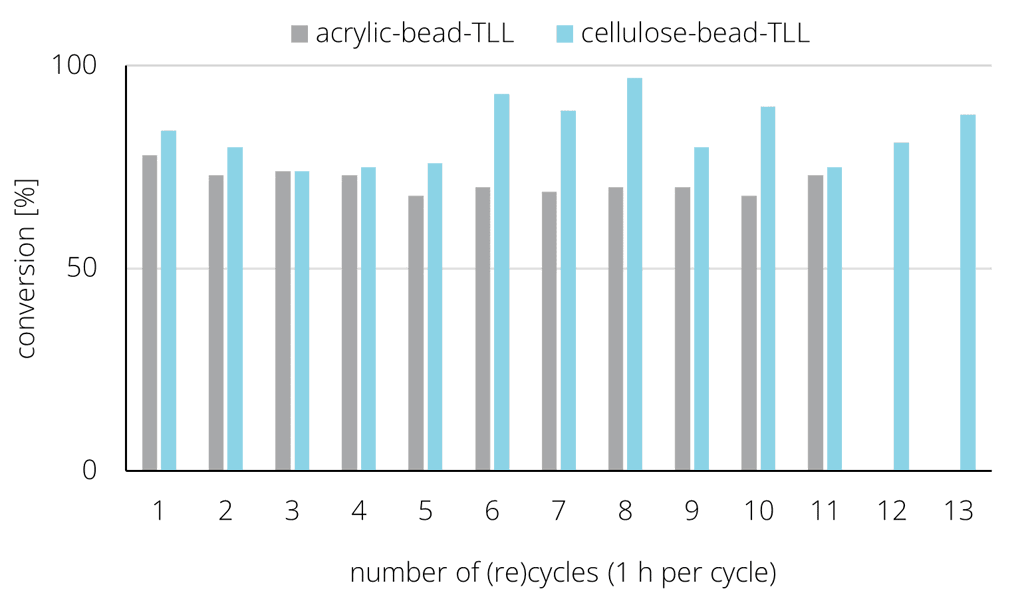
Additionally, the compatiblity and performance of 7 different lipases with the cellulose carriers was assessed for use in MCT oil hydrolysis. Interestingly, very different results were obtained: 3 lipases yielded excellent results (TLL, Eversa, Lipex), 2 performed moderately (Cutinase, PS) and 2 poorly (CALB, DF). This indicates underlying essential differences in the enzyme-support interaction, that are to be resolved next in order to grow understanding and to be able to further tailor enzyme carrier support properties.

If you want to stay up to date with developments by us and our collaboration partners in the Enzyme Technology Alliance, follow us on LinkedIn, or reach out to us for co-operations and access to our Newsletter!



