Pharmaceutical
The importance of modern medicine and pharmaceuticals is hard to overestimate. During the history of humankind, save for the last blink of an eye, one could expect a 50/50 chance of dying from an infection - grim odds. The situation with infections changed dramatically for the better from World War I and onwards. The major killers today: circulatory diseases, cancer, and respiratory diseases were of minimal concern for our hunter-gatherer ancestors due to a different lifestyle and life expectancy. However, modern medicine has made fantastic accomplishments to keep up with today’s need, and more effort and progress will be needed over the foreseeable future.
SpinChem can trace its roots back to the fine chemical and pharmaceutical space - starting out with transition metal catalysts before pivoting to develop the rotating bed reactor (RBR). Pharmaceutical industry was then the primary business area. And continues to be a very important area when developing the RBR. Today, biocatalysts have superseded chemocatalysts as the most used heterogeneous catalyst in RBR:s in Pharmaceutical industry.
From lab to production
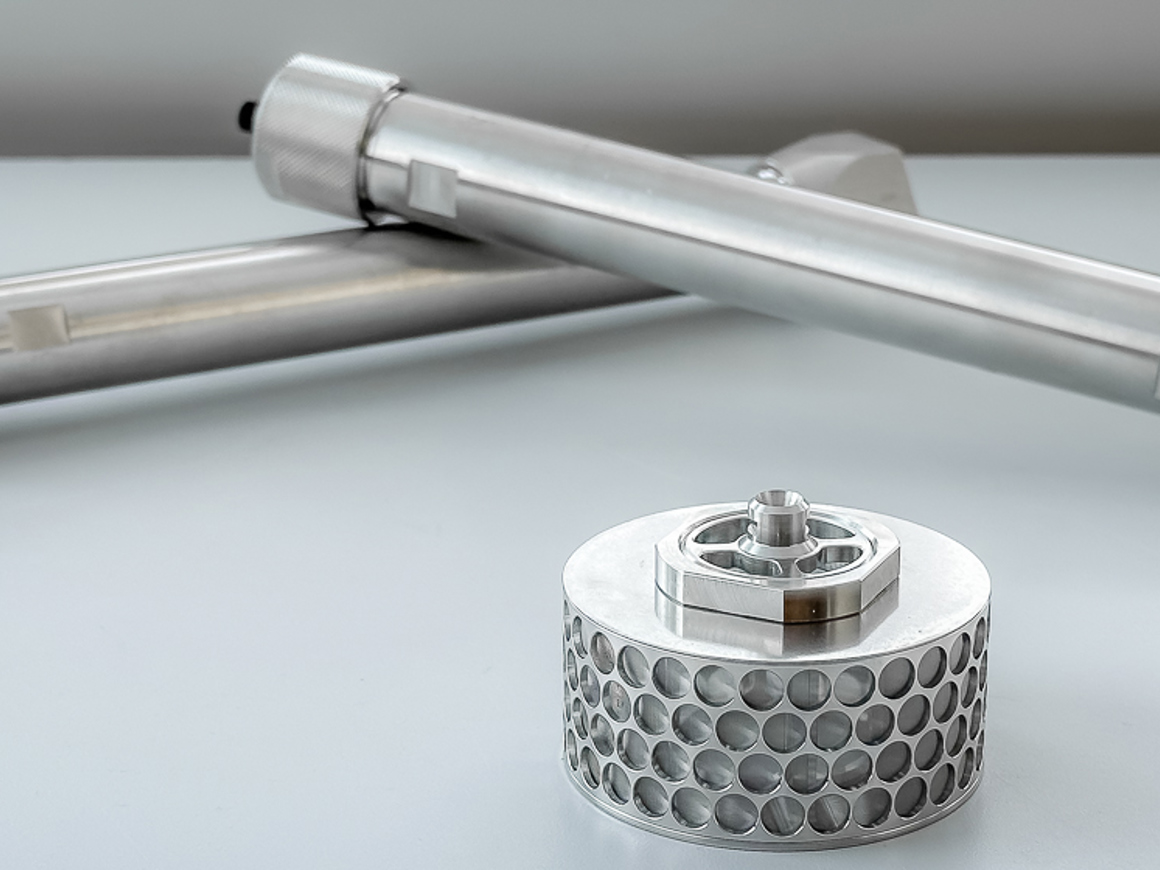
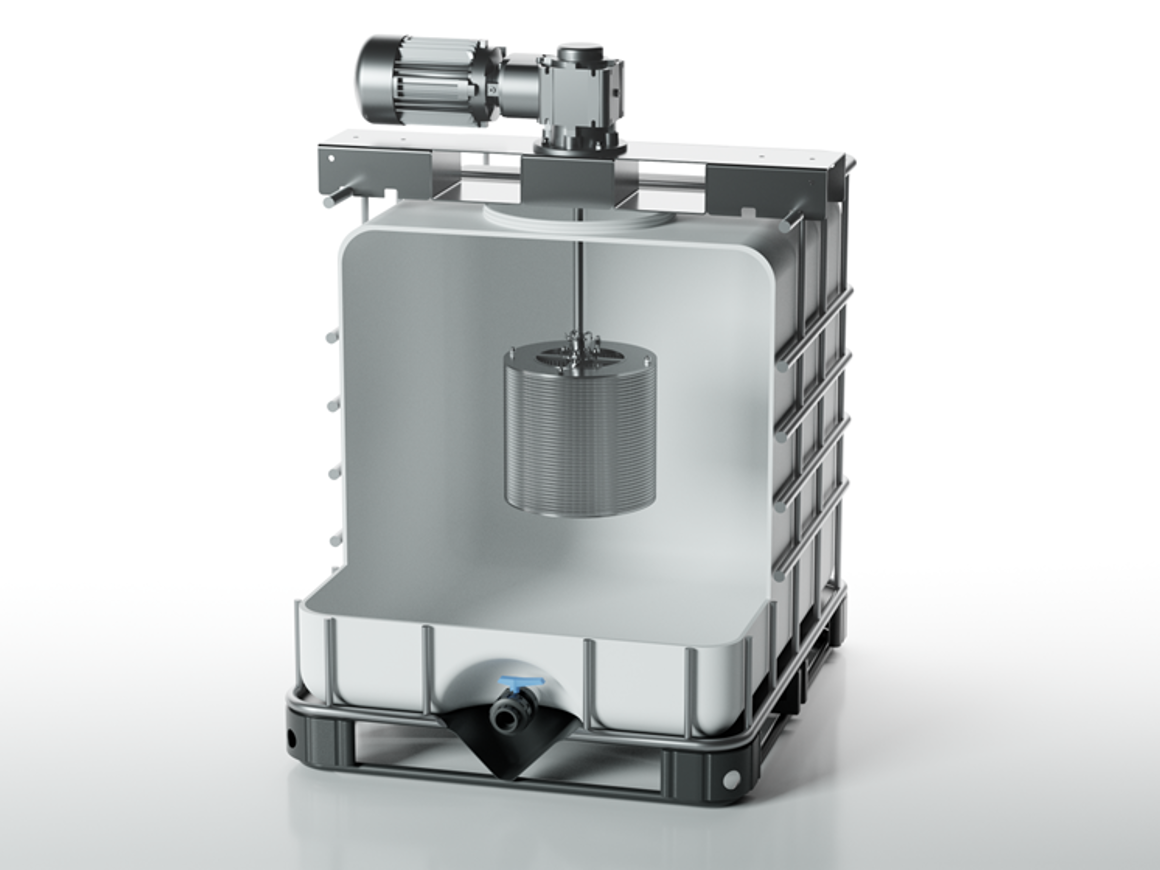
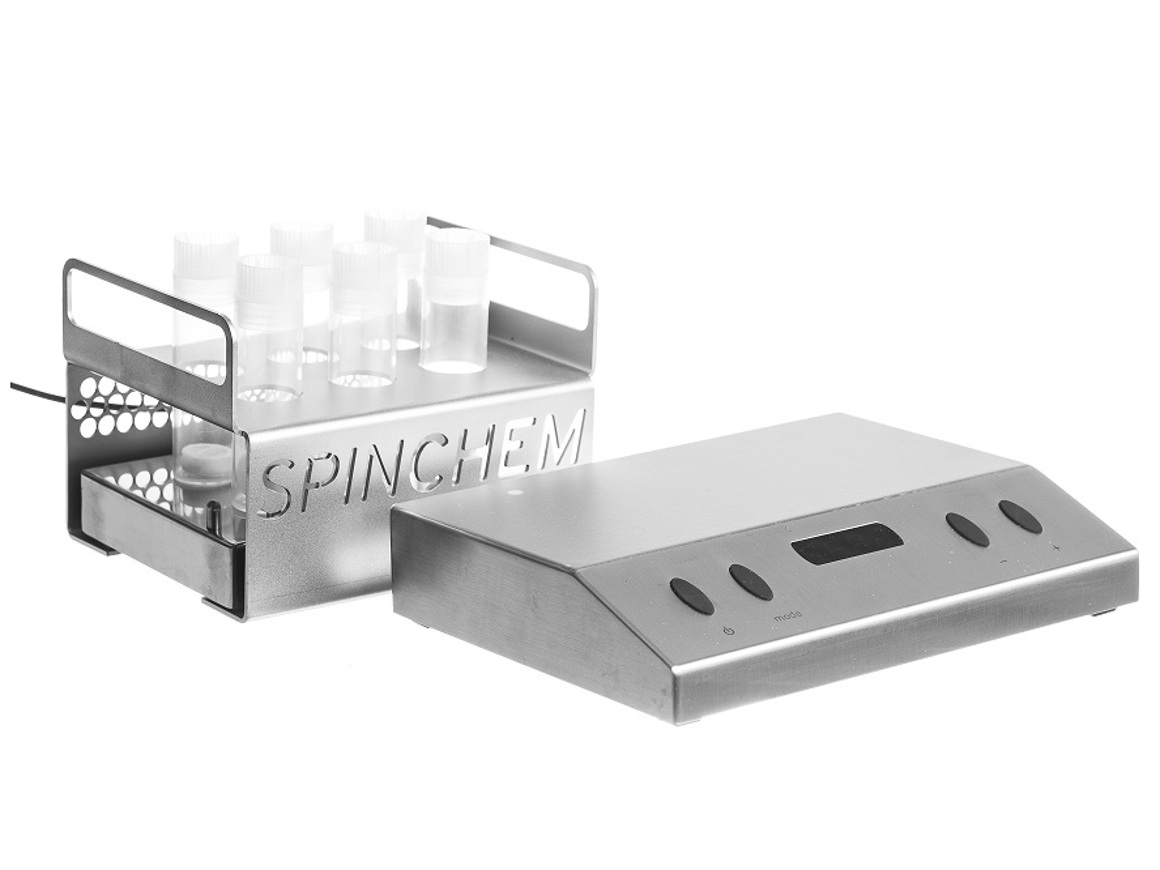
In the last few years, SpinChem has developed larger and larger RBR:s. As of today, there is nothing to indicate that there is an upper limit in size for the rotating bed reactor principle, consequently we will continue to develop even larger models as time and demand permits. Currently, the range of RBR models goes from 28 ml to 100 L solid phase. The corresponding liquid phase volume is completely application dependent and thus not listed for the RBR models larger than lab scale. The desired solid-to-liquid ratio will govern what size RBR is suitable, anywhere from tenths of percent to fractions of one percent has been used. When using heterogeneous catalysts, the activity of the catalyst and allowed reaction time will determine the loading. Similarly, when using adsorbents, allowed time is a factor as well as the capacity of the adsorbent in relation to the amount of solutes.
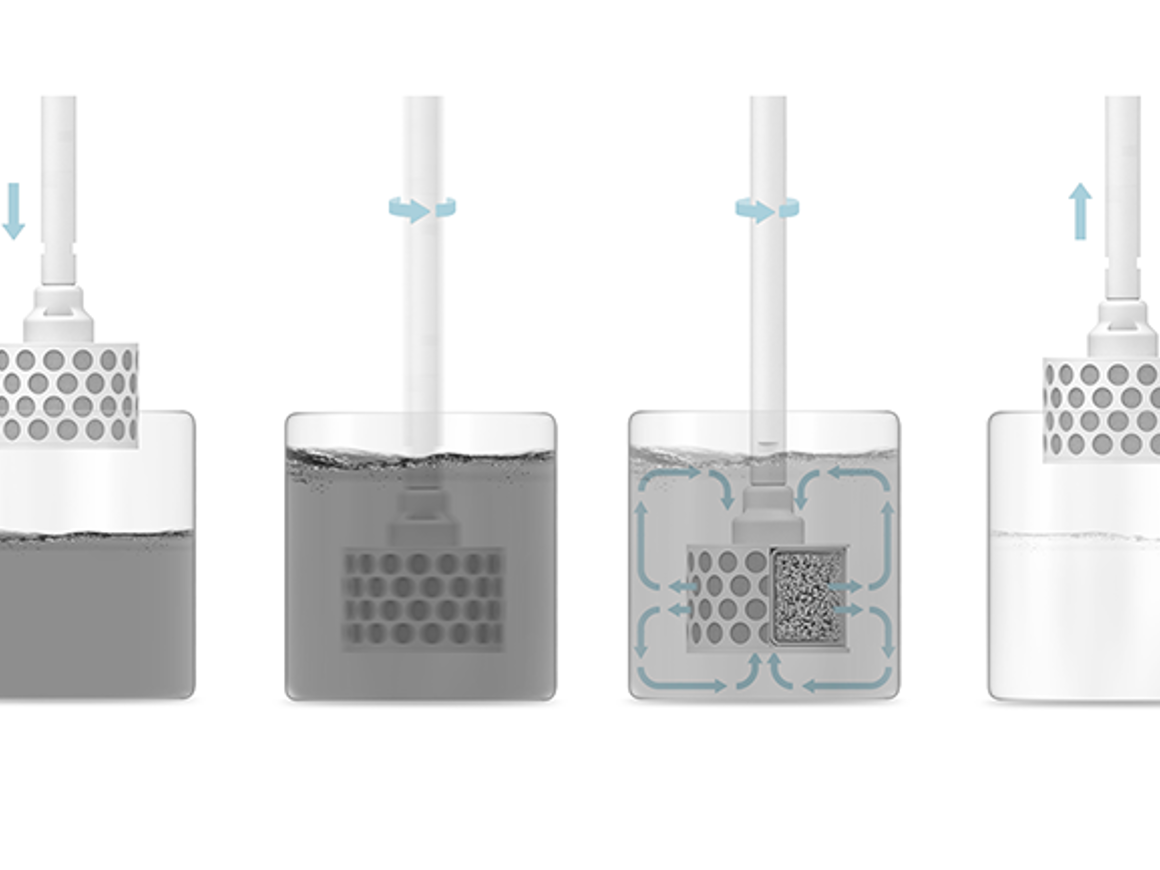
In the vessel
Most of the organic chemistry reactions and processes that are known may be relevant tools in the toolbox for the development and production of small-molecule pharmaceuticals. RBR technology may be used in the production of biomolecule-based therapeutics, but it will be discussed elsewhere. Briefly, an RBR can be used in these categories of processes: biocatalysis, chemocatalysis, non-catalytic reactions including solid-phase peptide synthesis, and work-up/ downstream processing.
Biocatalysis
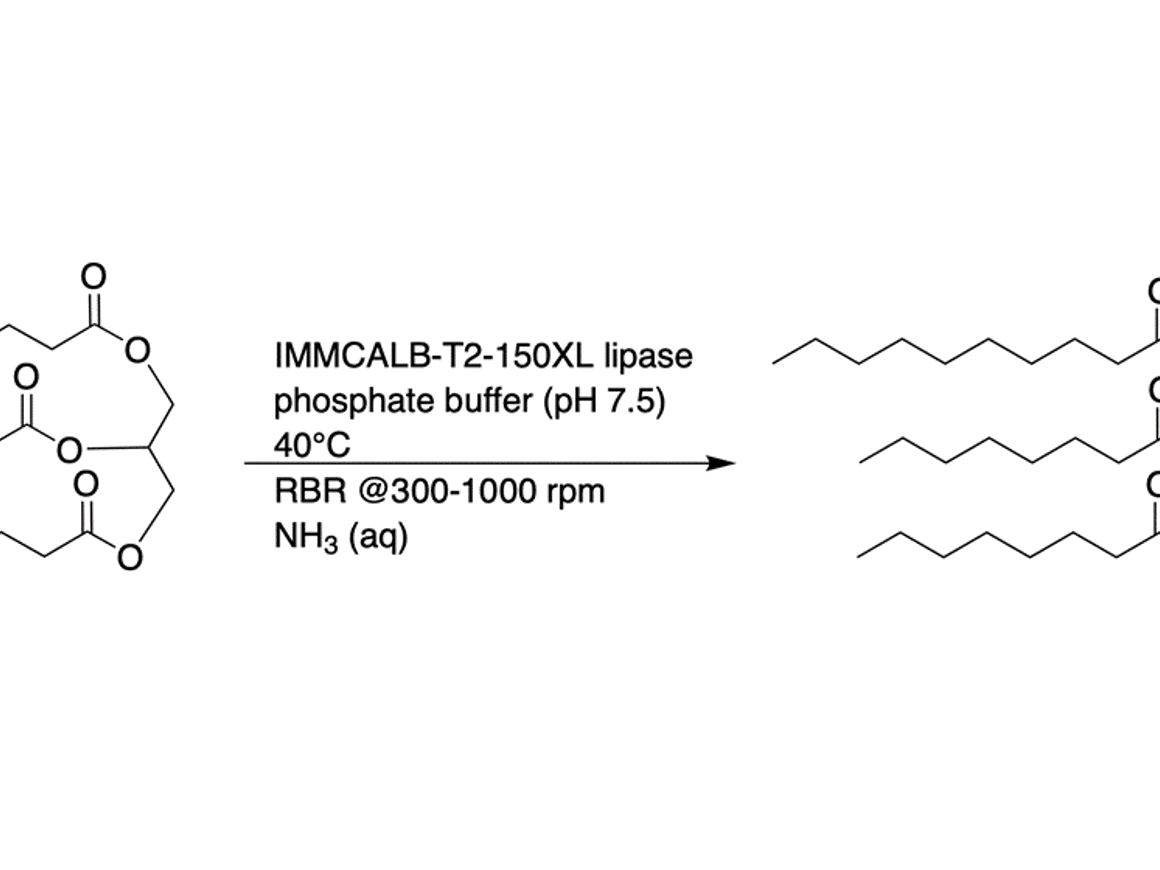
The selection of commercially available enzymes and possibilities to acquire custom-developed enzymes has exploded in the last few years. Enzymes from many classes are available in immobilized form, e.g. peptidases/amidases, lipases/ esterases, oxidoreductases, and aminotransferases. The activity and stability under non-physiological conditions is remarkable in many cases, which has allowed to make them a relevant or even superior alternative to classical chemical catalysis in commercial processes. In Pharma, perhaps the high regio- and stereoselectivity that enzymes may offer are even more interesting properties allowing cleaner reaction steps or alternative routes relative to chemical synthesis.
Chemocatalysis
Classical chemical catalysis has not stood still either. Saying that the emergence of transition metal catalysis has revolutionized the field is probably not overstating it. Named reactions such as Suzuki, Stille, Grubbs, Heck, Negishi, Sonogashira, and Grignard to name a few have been around long enough to be considered parts of an organic chemist’s basic toolbox. With immobilized transition metal complexes, these reactions are available in RBR, e.g. our partner Reaxa offers alternatives in their EnCat line.
A more recent development is seen with organocatalysis, which was awarded the Nobel Prize in chemistry in 2021. Organocatalysts are available in immobilized form, we look forward to the first application in an RBR.
Perhaps the most straight-forward heterogeneous chemocatalyst is ion-exchange beads for acidic and basic catalysis.
The final example of chemocatalysis here, and also the most requested is palladium-catalyzed hydrogenation. Regular Pd/C is in powder-form and too fine for an RBR. However, there are Pd(0)-catalyst and granulated Pd/C to use from different suppliers. SpinChem currently does not offer pressurized hydrogenation vessels, which does not prevent a customer from installing an RBR in such a vessel. Also, transfer hydrogenation has been proven to work in RBR, offering a convenient and safe alternative to hydrogen gas.
Stoichiometric reagents
As for non-catalytic applications of RBR, the most common are ion-exchange beads and drying by molecular sieves. There are, however, a large selection of commercially available immobilized reagents. As far as we know, immobilized reagents are yet to be utilized in an RBR. Presumably, the relatively high cost per capacity offered is preventing widespread use. For the right application, the cost may be recouped in easier downstream processing.
Solid phase peptide synthesis
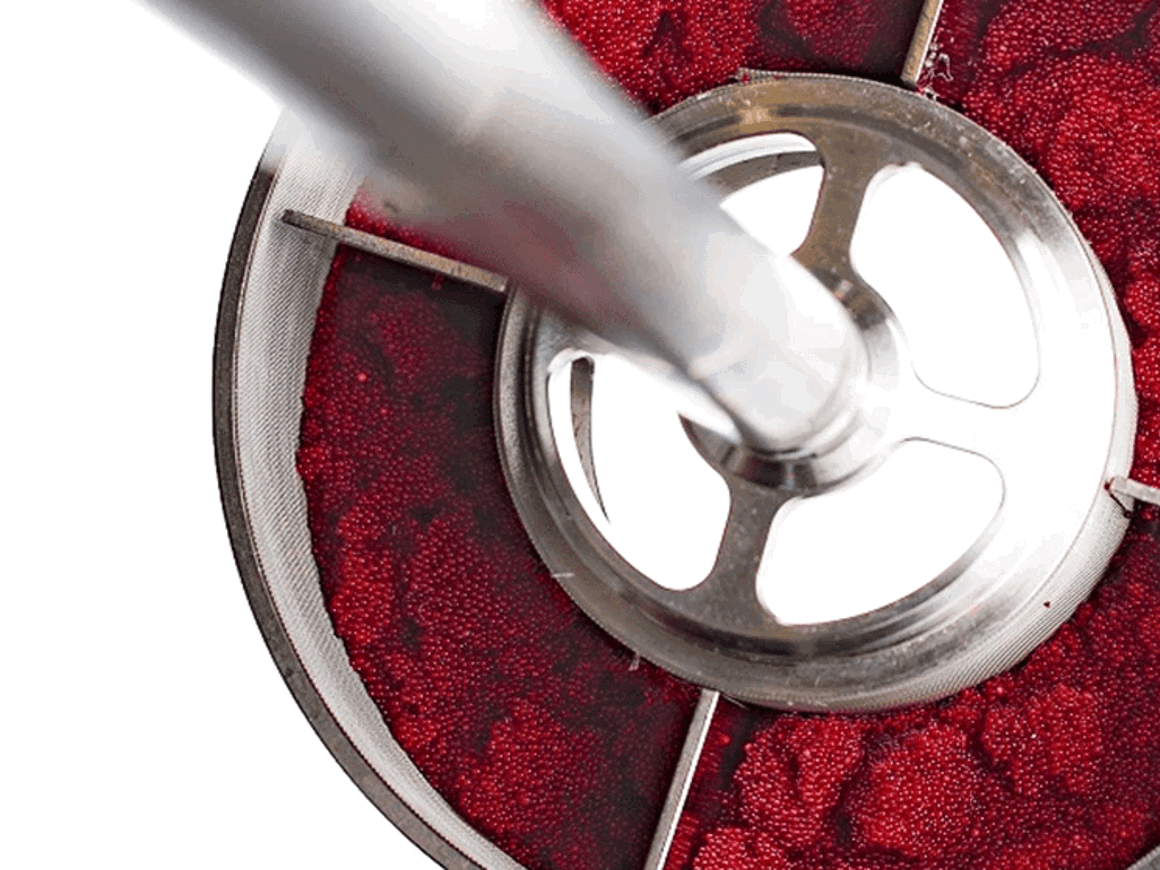
Carrier beads, such as Merrifield, Wang, and more recent developments, for solid-phase peptide synthesis may be considered a case of stoichiometric reagents suitable for RBR. The possibility to save solvent in these processes are obvious by the improved liquid flow rates through the packed bed compared to other technologies.
Downstream processing
Transition-metal catalysis was mentioned above. There is a risk that a small amount of the immobilized metal catalyst has leached into the solution during reaction. A good method to remove the often toxic heavy metal ions from the reaction product is a metal scavenging resin. The selection of heterogeneous scavengers is good, ranging from neutral to IEX-based, organic to inorganic backbone, and more general to more specific.
Perhaps less common in use, there are scavengers for selected organic compounds based on complementary electrophilic-nucleophilic reactivity, e.g. aldehyde-amine and Michael acceptor-thiol.
Adjusting pH during workup is a very common procedure and IEX resins are an option for doing this, also offering a way to produce less salt in the vessels during workup.
We would like to include treatment of waste streams in the context of downstream processing. It may make environmental and/or economical sense to treat your own waste streams locally before discharge or destruction. At the production site, the waste streams are contained, concentrated, and this is likely where the most knowledge about the content is found. Where large, costly, or specialized treatment equipment is needed, it may make more sense to transport to a common waste treatment site. Whether the waste stream contains excess dye from clothing manufacture or API precursors from pharmaceuticals production, a simple activated carbon treatment may be sufficient to make the waste stream acceptable for discharge. Best case scenario environmentally and financially, successfully treating the waste stream whether it is water or organic solvent, will allow it to be re-used in the process.
Ideally, the waste streams can be turned into a resource by extracting residual amounts of valuable components. Two examples comes from the pharmaceutical industry: scavenging and re-use of transition metals (e.g. palladium, iridium) from reaction waste after chemical TM-catalysis and recovery of API:s and precursors to API:s in small, residual concentrations from reaction waste or purification step waste. In both examples, the transition metal and API precursor is likely to be toxic in the wrong place (e.g. aquatic life) but valuable when recovered in pure form.